How Can Someone Blow the Whistle on Pharmaceutical Fraud?
It’s becoming increasingly commonplace for a pharmaceutical manufacturer or a pharmaceutical company to engage in fraudulent activities, such as falsifying the data behind the new drug application, engaging in kickbacks and/or manipulating the marketing during the distribution of its prescription drugs, which can be considered off label marketing or off label promotion. All of these activities not only undermine federal and state law but also jeopardizes patient health and are treated very seriously under the law.
While it may seem challenging to take on wealthy drug manufacturers, a pharma whistleblower can fight corruption and protect themselves with the aid of a pharmaceutical fraud lawyer, like the whistleblower law firm of Brown, LLC who has a track record of success in battling big pharma
A pharmaceutical attorney will guide the whistleblower through the process of taking legal action against these unlawful practices, providing the individual with field vision about what to expect if interested in filing a claim, how to best protect against retaliation and if the case succeeds helping them secure a pharmaceutical whistleblower reward for helping the government.
Some of the largest whistleblower awards over the years stem from pharmaceutical whistleblowers who use the False Claims Act and other statutes to blow the whistle on unlawful practices such as kickbacks for prescriptions, off-label promotion or other schemes to defraud programs like Medicare, Medicaid or private insurance in certain states like California and Illinois. To blow the whistle on pharmaceutical fraud using the False Claims Act you must file the qui tam lawsuit with a whistleblower law firm and, of course, a pharmaceutical fraud lawyer will assist you in determining whether it’s worth filing the case at all and go over the risks and rewards. Hundreds of millions of dollars have been given as pharmaceutical whistleblower awards over the years, which will spill over to billions in whistleblower rewards eventually, given the damages in these pharmaceutical schemes are sometimes stratospheric.
In the fight against pharmaceutical fraud, the role of a pharmaceutical fraud lawyer is indispensable, offering legal options and safeguarding a whistleblower from pharmaceutical company reprisal. A pharmaceutical fraud whistleblower attorney will work tirelessly to ensure whistleblowers are supported every step of the way, from filing qui tam lawsuits to placing the case in the best possible light to try and obtain an award for the work.
With billions at stake in potential damages and rewards, the decision to report healthcare fraud is made easier with the right pharma fraud law firm and the understanding about the various protections under the law.
What is Pharmaceutical Fraud?
The U.S. Food and Drug Administration (“FDA”) enforces strict requirements on pharmaceutical products to ensure their safety and efficacy on behalf of consumers. Unfortunately, pharmaceutical companies sometimes abuse the system to cut costs and increase profits or because they’re under pressure to deliver the next best pharmaceutical product. Government programs such as Medicare, Medicaid, and TRICARE often foot the bill for illegally distributed and marketed products, costing taxpayers billions of dollars each year.
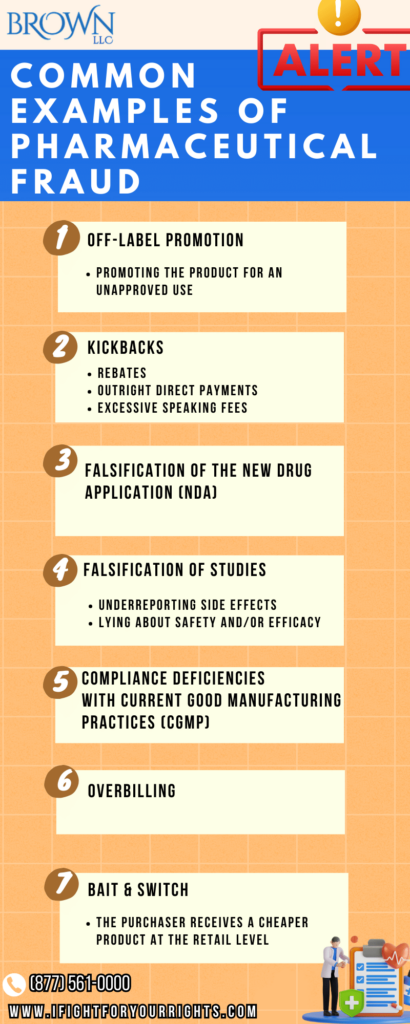
The Most Common Pharmaceutical Fraud
- Pharmaceutical fraud can take many different forms, such as:
- Off-label promotion – off-label marketing
- Promoting the product for an unapproved use
- Promoting the product in a way that wasn’t explicitly approved
- Engaging in kickbacks for prescriptions, such as:
- Free vacations
- Excessive speaking fees based on volume of scripts
- Opaque rebates
- Outright payments
- Falsification of the new drug application (NDA)
- Falsification of studies
- Over-disqualification of control group people with side effects
- Underreporting side effects
- Lying about safety and/or efficacy
- Serious compliance deficiencies with Current Good Manufacturing Practice (cGMP)
- Overbilling
- Bait & Switch in which the purchaser receives a cheaper product at the retail level
The Importance of Whistleblowing in the Pharmaceutical Industry
In the past certain pharmaceutical products deliberately downplayed the risks of their products, such as pulmonary embolism incidents, or its link to cancers, and consequently, physicians and consumers could not make educated decisions about whether the benefit of the product outweighed its side effects. People died. People suffered strokes and other embolic events. Blowing the whistle on pharmaceutical fraud can save lives and educate the public about the real risk. Further, if the downplaying of the risks is so profound that the government and other insurers wouldn’t have purchased the product had they known the truth, then the insider may have significant rights.
When the products are purchased by a government insurance program like Medicare or Medicaid or Tricare, it is considered pharmaceutical Medicare fraud if the company conceals quality issues or approval issues or cGMP issues. This fraud can be remedied through the False Claims Act whistleblower statute. Here, an FDA whistleblower plays a crucial role, using the Federal False Claims Act to report pharmaceutical fraud that has been hidden within opaque corporate policies. Compound that with the ability to allege that the pharmaceutical company may have fundamentally lied to its shareholders in falsely stating that a product was approved or is passing quality inspections when it’s not, and the insider has the ability to file an SEC whistleblower case anonymously with the use of an SEC whistleblower law firm in addition to the underlying False Claims Act case to address the pharma fraud
Examples of Pharmaceutical Fraud and Their Consequences
Examples of pharmaceutical fraud can include:
- Marketing a drug for a use that has not been approved by regulatory authorities – off-label promotion
- Concealing or downplaying the risks associated with a drug
- Offering bribes or kickbacks to healthcare providers to encourage them to prescribe a particular drug
- Falsifying data in clinical trials to make a drug appear more effective or safer than it actually is
- Engaging in price gouging or other anti-competitive practices
Pharmaceutical fraud can have serious consequences for public health, as it can lead to the use of unsafe or ineffective drugs, unnecessary healthcare costs, and actual harm to the patients or lead patients to rely on a product that’s not working when they could be using more effective alternatives.
Falsification of the FDA Approval Process for New Drugs
One type of pharmaceutical fraud is the falsification of the FDA approval process for a New Drug Application (NDA). This is prohibited and can dovetail into a mass tort when people are injured by the signature injuries that were suppressed in the initial application. There is always some degree of gamesmanship when applying for an NDA and some techniques inhibit transparency. For example, some companies deliberately run their product testing on people in countries in which there are privacy laws that hinder a full audit trail. Other times, the company overly scrutinizes the individuals with side effects and does not subject the rest of the control group to the same scrutiny. For example, if someone suffers a pulmonary embolism from a newly tested birth control product, they may argue that they should be excluded from the sample since they then discovered their grandmother had a blood clot event, but not look to disqualify those that didn’t suffer side effects who had a family history of clotting which therefor throws off the objectivity and tilts the study.
Adulteration and Quality Control Issues in Pharmaceutical Manufacturing
Another type of pharmaceutical fraud process is adulteration and quality control. The manufacturers must maintain and adhere to CGMP. CGMP is the Current Good Manufacturing Practice regulations enforced by the FDA. Testing methods to audit the quality of the drugs are agreed upon through the approval process with the FDA. If the company deviates from the agreed-upon testing method, it could trigger liability. If the company adulterates the product to pass agreed-upon testing, it could trigger liability and if the company releases quarantined lots without retesting, there may be liability. Falsification of the results without testing, or with a failed test also are grounds to hold companies liable. A consistent theme amongst all the whistleblower streams is that the maintenance of a reliable audit trail is critical to ascertain what happened when, and the destruction of the trail may lead to certain negative inferences against the company.
Pharmaceutical False Claims Act Settlements
Pharmaceutical False Claims Act Settlements have greatly impacted the industry through the combined efforts of a pharma fraud attorney working alongside FDA whistleblower pharmaceutical insiders. In fact, some of the biggest False Claims Act settlements have been insiders, including sales representatives, who blow the whistle on off-label promotion of a product or know about kickbacks. Off-label promotion or off-label marketing is when the product was approved for one medical use, but there may be indicia that has not been fully vetted about another quality of the product that is them marketed to individuals in the unapproved manner. As an example, GlaxoSmithKline agreed to pay $3 billion to settle FCA claims related to off-label promotion, false claims, and other allegedly illegal practices. The settlement included $1 billion in criminal fines and forfeiture and $2 billion in civil damages, making it one of the largest healthcare fraud settlements in history which also entitled the whistleblowers to a whistleblower reward exceeding $100 million.
Off-Label Marketing
When a pharmaceutical product or medical equipment is prescribed for a use or purpose that has not been cleared by the FDA, that is called off-label use. Off-label use of drugs and other products poses safety concerns because the product was likely not rigorously tested for that use. Federal law prohibits companies from promoting unapproved, off-label uses that have not been approved and demonstrated to be safe and effective. Despite this, many pharmaceutical companies resort to promoting off-label uses to increase revenue and market share, which puts profits before people. The product becomes snake oil where in the past certain companies would make outlandish claims about the unapproved uses of the product, such as curing acne, helping with weight loss, or creating a new vigor, however, without testing the product explicitly for that purpose the efficacy is questionable, and what’s worse, the patient is subjected to the off label use with potential side effects but not approved study to support the off label use. Any medicine has its own risks and why take it if the utility of using it is dubious.
If the pharmaceutical company induces or encourages off-label uses in their marketing or promotional materials or sales methods, that may give rise to a violation of the False Claims Act.
Pharmaceutical Kickbacks
Financial kickbacks to medical providers often go hand-in-hand with off-label use and other common types of pharmaceutical fraud. By providing items of value to medical providers, pharmaceutical companies can generate lucrative prescriptions for their products regardless of whether they are safe or appropriate for the receiving patients. Although sometimes it’s just brazen with inducements of pay to play (prescribe), other times the kickbacks take a much more subtle form.
Kickbacks to medical professionals can take the form of cushy and well-paid consulting and seminar gigs, travel and vacation opportunities, free or discounted products and services, and sometimes cold hard cash. The Federal Anti-Kickback Statute strictly prohibits the provision or receipt of anything of value in exchange for the prescription of any drug or medical product. A violation of the Anti-Kickback Statute is, in turn, a violation of the False Claims Act, which rewards whistleblowers for successful prosecution.
Even outside of the pharmaceutical context, kickbacks are all too commonplace in the medical community. Read more about kickbacks in healthcare here.
Reporting Pharma Fraud in Healthcare the Right Way
Over the years, whistleblowers have helped the government recover billions of dollars by reporting illegal schemes perpetrated by pharmaceutical companies. These whistleblowers helped to protect patients, taxpayer dollars, and the integrity of the healthcare system.
Pharmaceutical insiders who witness illegal marketing or other kinds of fraud can provide an important public service and receive financial rewards by blowing the whistle the right way. However, if you blow the whistle the wrong way without consulting an experienced pharmaceutical fraud lawyer, your case could be over before it begins. To understand your rights as a pharmaceutical whistleblower, you should consult with our qui tam team by calling (877) 561-0000 for a free, confidential consultation.